The Sec 3 Chemistry Wake-Up Call: Your O-Level Prep Starts Now
- Master challenging Chemistry concepts before they become O-Level nightmares
- Transform abstract topics like electrolysis into understandable, visual processes
- Build connections between chapters that boost your A1 potential
- Develop smart study habits that eliminate careless errors under exam pressure
The phone calls always start the same way: "My child was doing fine in Sec 2 Science, but now they're struggling with Sec 3 Chemistry. Is this normal?"
After working with hundreds of families navigating Singapore's education system, I can tell you – you're not alone. The transition from integrated science to pure Chemistry represents one of the steepest learning curves in our curriculum. What makes it particularly challenging is that students often don't realize they're falling behind until it's almost too late.
According to MOE's latest curriculum guidelines, Sec 3 Chemistry introduces concepts that form 60% of your O-Level examination content. This means that struggling students can't afford to "figure it out later" – the foundation needs to be solid now.
The Sec 3 Reality Check: Why Chemistry Feels Impossible
The jump from Sec 2 Science to Sec 3 Chemistry isn't just about difficulty – it's about completely different thinking skills. In lower secondary science, you could succeed by memorizing facts and following procedures. Chemistry demands something entirely different: you need to visualize invisible processes, understand cause-and-effect relationships, and apply principles to unfamiliar scenarios.
Here's what I've observed over the past decade: students who struggle most with Sec 3 Chemistry typically hit walls in three specific areas:
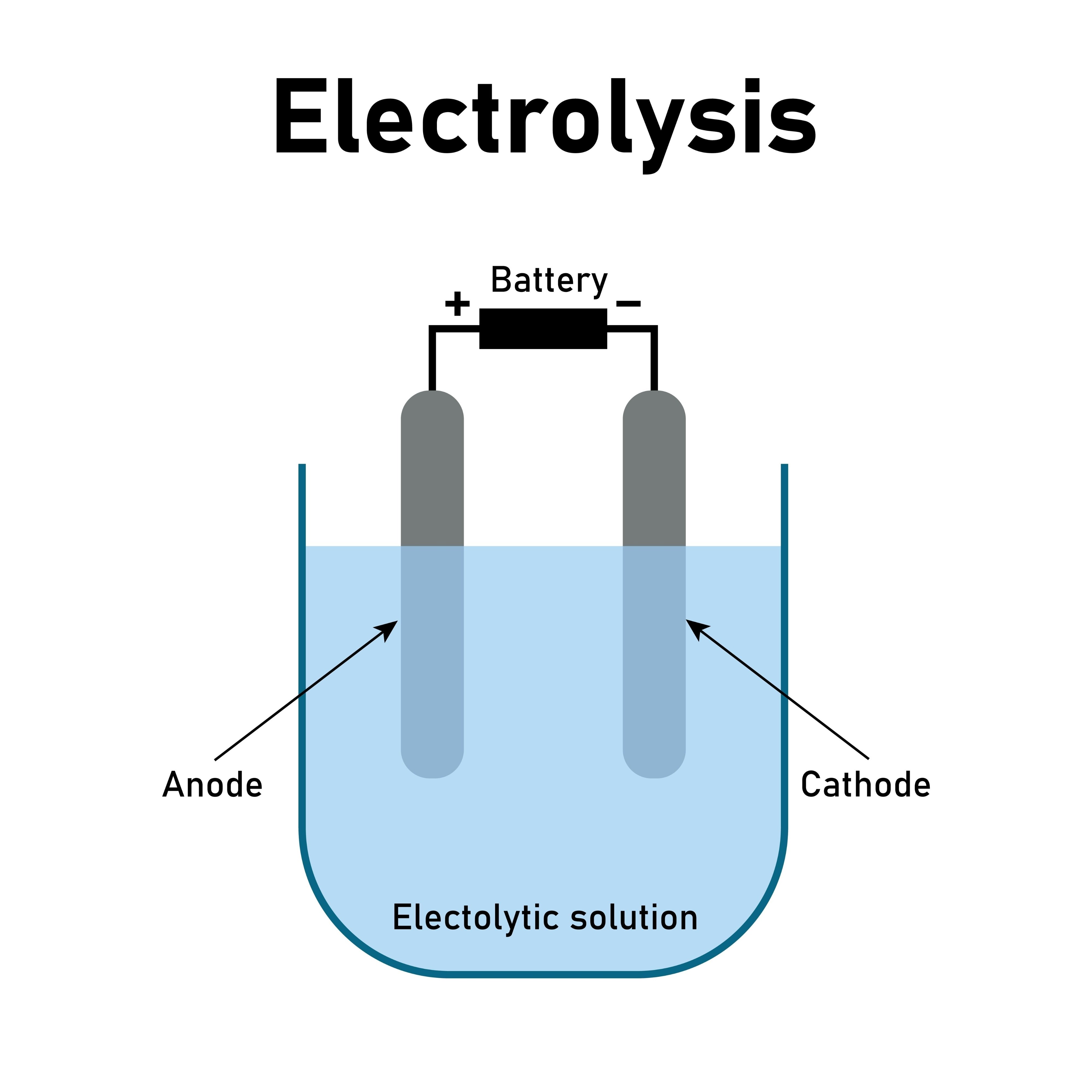
Electrolysis – The abstract nature of ion movement and electron flow confuses students who try to memorize instead of understand. They can recite "AN OX, RED CAT" but panic when asked why copper ions discharge before hydrogen ions in aqueous solutions.
Moles and Stoichiometry – The mathematical precision required here catches students off-guard, especially when combined with other concepts in complex calculations.
Organic Chemistry – The sheer volume of reactions and structural formulas overwhelms students who haven't developed proper organizational systems.
The thing is, these aren't insurmountable obstacles. They're predictable challenges that can be systematically addressed with the right approach. But here's the critical point: O-Levels are just one year away, and according to SEAB examination statistics, students who enter Sec 4 with unresolved Sec 3 gaps rarely achieve their target grades.
This December holiday period represents your last real opportunity to build a strong foundation before Sec 4 content accelerates. You cannot build advanced Chemistry understanding on shaky Sec 3 foundations.
Stop Memorizing, Start Understanding: The Foundation Fix
The biggest trap I see students fall into is treating Chemistry like a memory subject. They create flashcards for every equation, memorize mnemonics for every process, and wonder why they can't handle application questions during exams.
Here's a real example from recent O-Level papers: Students memorized that "chlorine gas is produced at the anode during electrolysis of sodium chloride solution." But when the question asked them to explain what would happen if the concentration changed, or if a different electrode material was used, they were completely lost.
The memorization approach fails because O-Level Chemistry questions are designed to test understanding, not recall. SEAB's assessment objectives explicitly state that 40% of marks come from "application and analysis" – skills that require deep conceptual understanding.
The Understanding Approach:
Instead of memorizing that "chloride ions discharge at the anode," ask yourself:
- Why do ions move toward electrodes in the first place?
- What forces are acting on these charged particles?
- Why would one ion discharge before another?
- How does the Electrochemical Series determine this preference?
💡 Pro Tip: Every time you encounter a Chemistry fact, challenge yourself to explain the "why" behind it. If you can't explain why something happens, you don't truly understand it yet.
When you understand that oppositely charged particles attract each other, that ions have different tendencies to gain or lose electrons, and that ease of discharge follows predictable patterns, suddenly electrolysis becomes logical rather than mysterious.
Practice This Understanding Method:
- Identify the underlying principle – What fundamental concept explains this observation?
- Connect to bigger patterns – How does this fit with other Chemistry principles you know?
- Predict new scenarios – If you changed one variable, what would happen and why?
- Explain to someone else – Can you teach this concept clearly without referring to notes?
This approach takes more initial effort than memorization, but it pays massive dividends during O-Level examinations when you encounter unfamiliar scenarios that test your ability to apply principles rather than recall facts.
Visualize the Invisible: Making Abstract Concepts Concrete
Chemistry's biggest challenge is that most processes happen at a scale we can't see. Ions migrating, electrons flowing, bonds forming and breaking – these are invisible processes that your brain struggles to grasp without visual aids.
Many students tell me they "understand" electrolysis after reading their textbook, but when I ask them to sketch what's actually happening inside an electrolytic cell, they realize their understanding is much shakier than they thought.

The Visualization Strategy:
For every abstract Chemistry concept, create a visual representation that shows:
What's moving – Draw arrows showing ion migration, electron flow, or molecular motion
What's changing – Use different colors or symbols to show before/after states
What's causing the change – Label forces, energy inputs, or driving factors
Electrolysis Example:
Instead of memorizing "cations move to cathode, anions move to anode," draw it:
- Sketch the electrolytic cell with electrodes clearly labeled
- Draw positive ions (Cu²⁺, H⁺) moving toward the negative cathode
- Draw negative ions (SO₄²⁻, OH⁻) moving toward the positive anode
- Show electrons flowing through the external circuit
- Illustrate discharge reactions at each electrode
This visual approach transforms abstract concepts into concrete processes your brain can work with. When exam questions present unfamiliar scenarios, you can sketch out what's happening rather than panic about what to memorize.
Beyond Electrolysis:
This visualization technique works for every challenging Sec 3 topic:
- Moles: Draw particle diagrams showing actual numbers of atoms/molecules
- Organic Reactions: Sketch structural changes step-by-step
- Acids and Bases: Illustrate proton transfers and ion formations
Students who consistently use visual learning methods show significantly better performance on application questions – exactly the type that differentiate A1 from B3 grades.
Connect the Dots: Why Isolated Chapter Study Fails
One of the biggest mistakes I see students make is treating each Chemistry topic as a separate, unrelated unit. They master electrolysis in Chapter 8, understand moles in Chapter 6, but panic when O-Level questions combine these concepts.
Here's the reality: Recent O-Level Chemistry papers consistently feature questions that require you to integrate knowledge across multiple chapters. The ability to make these connections often determines whether you achieve your target grade.
Real O-Level Integration Example:
A typical challenging question might ask you to:
- Predict the products of electrolysing copper sulfate solution (Electrolysis knowledge)
- Calculate the mass of copper deposited after 30 minutes (Moles and Stoichiometry)
- Explain why the blue color fades during the process (QA and Color)
- Suggest what happens if you reverse the current (Application thinking)
Students who learned these topics in isolation struggle because they haven't practiced thinking across chapter boundaries. They might know each individual concept but can't orchestrate them together under exam pressure.
The Connection Strategy:
As you review each topic, actively look for links to other chapters:
Electrolysis connections:
- Links to Moles: Calculate quantities of products formed
- Links to Ionic Theory: Understanding which ions are present
- Links to Reactivity Series: Predicting discharge preferences
- Links to Acids and Bases: pH changes during electrolysis
Moles connections:
- Links to Chemical Formulas: Understanding molecular formulas
- Links to Stoichiometry: Calculating reaction quantities
💡 Pro Tip: Create a "connections map" for each major topic. Draw lines showing how it relates to other Chemistry concepts you've learned.
Practice Integration Early:
Don't wait for Sec 4 to practice integrated questions. During this holiday period, specifically seek out problems that combine multiple concepts. This builds the mental flexibility you'll need for O-Level success.
Students who master concept integration typically score one full grade higher than those who learn topics in isolation. The difference between understanding Chemistry as connected principles versus isolated facts often determines A1 versus B3 outcomes.
Practice Smart, Not Just Hard: The Strategic Advantage
Working with motivated Singapore families, I often see students putting in tremendous effort – hours of daily practice, multiple assessment books completed, tuition classes attended religiously. Yet their Chemistry grades remain stubbornly disappointing.
The issue isn't effort; it's strategy. Mindlessly completing question after question without reflection builds false confidence while reinforcing the same mistakes repeatedly.
The Smart Practice Method:
Every time you get a question wrong, invest time in deep analysis:
Step 1: Categorize the Error
- Conceptual Gap: Did you misunderstand a fundamental principle?
- Careless Mistake: Did you know the right approach but make a silly error?
- Application Failure: Did you struggle to apply knowledge to an unfamiliar scenario?
- Integration Challenge: Did you fail to connect different concepts appropriately?
Step 2: Address the Root Cause
- For Conceptual Gaps: Go back to basics, rebuild understanding from first principles
- For Careless Mistakes: Develop systematic checking procedures
- For Application Failures: Practice more varied scenarios with the same underlying concept
- For Integration Challenges: Work on connecting different topics explicitly
Step 3: Document Patterns
Keep a "mistakes journal" that tracks:
- Which types of errors you make most frequently
- Which topics generate the most mistakes
- Which question formats consistently trip you up
- Which checking strategies help you catch errors
📚 Key Insight: Students who analyze their mistakes systematically improve 2-3 times faster than those who simply repeat practice questions.
The Pre-Exam Mental Checklist:
Smart practice builds automatized checking habits that work under pressure:
- Units Check: Are my units consistent and correct?
- Magnitude Check: Does my numerical answer make logical sense?
- Concept Check: Have I applied the right principle for this scenario?
- Completeness Check: Have I answered all parts of the question?
Developing this systematic approach during Sec 3 creates habits that prevent costly errors during actual O-Level examinations. Research shows that systematic error-checking can improve exam performance by 10-15% – often the difference between achieving your target grade and falling just short.
Your December Action Plan: Don't Waste This Critical Window
This holiday period is your last real opportunity to fix Sec 3 foundation issues before Sec 4 content accelerates. Here's exactly how to use this time strategically:
Week 1: Diagnostic Assessment
- Take practice questions from each major Sec 3 topic
- Identify which concepts you can explain confidently versus those you've only memorized
- Create a priority list focusing on topics that will be heavily tested in O-Levels
Week 2-3: Foundation Rebuilding
- Focus on your weakest topics using the understanding approach
- Create visual diagrams for abstract concepts
- Practice explaining concepts out loud or to family members
Week 4: Integration Practice
- Work on questions that combine multiple concepts
- Practice the smart error-analysis method
- Build your systematic checking habits
Week 5-6: Advanced Application
- Challenge yourself with unfamiliar scenarios
- Time yourself to build exam-appropriate speed
- Refine your mental checklist for error prevention
💡 Pro Tip: If you're struggling to self-diagnose your weaknesses or finding it difficult to build understanding independently, this is exactly when professional guidance makes the biggest difference. A diagnostic assessment can pinpoint precisely which concepts need rebuilding and provide a customized plan for your specific gaps.
The O-Level Reality: Why Foundation Matters More Than You Think
According to Singapore's education performance data, students who enter Sec 4 with solid conceptual foundations consistently outperform peers who rely primarily on memorization strategies. This isn't just about grades – it's about building the analytical thinking skills that Singapore's education system is designed to develop.
The Chemistry concepts you're learning now – understanding invisible processes, making logical connections, applying principles to new scenarios – these are exactly the problem-solving skills that will serve you in A-Levels, university, and beyond.
But here's what makes this holiday period so critical: once Sec 4 begins, you'll be juggling new content alongside O-Level preparation pressure. Students who haven't solidified their Sec 3 understanding find themselves constantly playing catch-up, never getting ahead of the curve.
Success Stories Without the Names:
After a decade of helping Singapore students navigate Chemistry challenges, I've seen consistent patterns. Students who use this holiday period to rebuild foundations typically:
- Improve their Chemistry grades by 1-2 levels within the first Sec 4 term
- Report feeling much more confident during O-Level preparation
- Achieve target grades that previously seemed out of reach
- Develop study skills that benefit them across all subjects
The key difference? They stopped trying to memorize their way to success and started building genuine understanding.
Ready to Transform Your Chemistry Understanding?
If you're reading this and recognizing your own Chemistry struggles – whether it's confusion about electrolysis, panic about moles calculations, or general uncertainty about "how to study Chemistry" – you're not alone, and more importantly, these challenges are completely fixable.
My background includes NTU Honours with Distinction and straight A's in both O-Level and A-Level Chemistry, but what matters more is my specialized focus on helping Sec 3 and Sec 4 students transition from memorization to understanding. Over the years, I've developed diagnostic tools that can pinpoint exactly which concepts need rebuilding and create customized plans for different learning styles.
The students who benefit most from professional guidance are those who:
- Put in effort but aren't seeing corresponding grade improvements
- Feel confused about abstract concepts like electrolysis or bonding
- Panic when Chemistry questions combine multiple concepts
- Want to build strong foundations now rather than struggle through Sec 4
This December holiday period represents your best opportunity to make real progress. Don't let another term pass feeling confused and overwhelmed.
Take the Next Step:
If you're ready to stop memorizing and start understanding Chemistry, book a free diagnostic consultation to identify your specific gaps and create a strategic plan for O-Level success. You can also explore our comprehensive Chemistry tuition services designed specifically for Singapore's curriculum requirements.
For additional learning resources and study tips, browse our education blog or read what other parents have shared in our testimonials section.
Remember: every Chemistry concept that seems impossible right now can become completely manageable with the right approach. Your O-Level success starts with the foundation work you do during this holiday period.
Ready to begin? Contact our education specialists today and transform your Chemistry understanding before Sec 4 begins.
Tags:
📚 Related Reading
Polytechnic Open Houses 2026 (Jan 8-10): The Ultimate Guide for O-Level Grads
All 5 Singapore polytechnics are opening their doors Jan 8-10, 2026 - your only chance to experience campus life before JAE applications close. Here's your complete guide to making the most of these crucial three days and choosing the right path for your O-Level results.
Why Starting Tuition in January Beats Waiting Until June: A Singapore Parent's Guide
The 'wait and see' approach costs Singapore students dearly. After helping 350+ families navigate our education system, here's why January tuition starts lead to better outcomes than June panic decisions.
DSA 2026: Why P6 and Sec 3 Students Must Start Preparing in January
The DSA 2026 application window opens in May, but successful students start building their portfolios in January. Here's your strategic guide to creating compelling evidence of talent and achievement before the rush begins.
Need Help with Your Studies?
Get personalized tutoring from The Singapore Syllabus expert team. Our experienced tutors are here to help your child excel in mathematics and science.











